Description
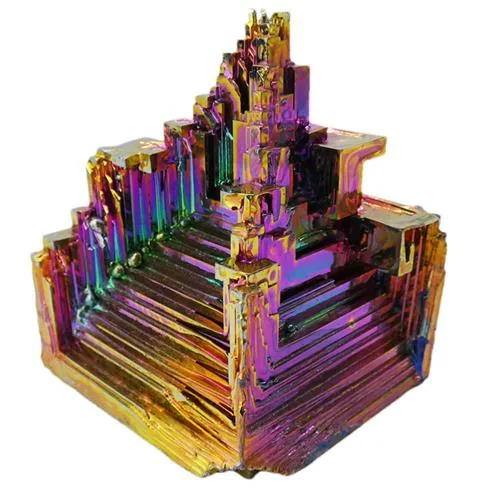
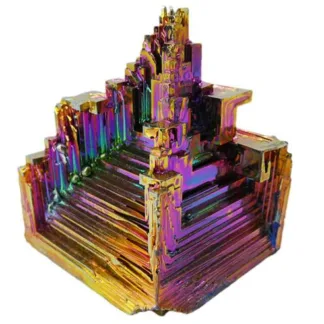
Bismuth Sample – Natural Bismuth – Lab Grown This bismuth specimen is large and weighs approximately 208g. Bismuth specimens are highly prized for their enchanting rainbow-like appearance, a stunning result of surface oxidation. When bismuth reacts with oxygen, a delicate oxide layer forms, creating a brilliant iridescent display of colours. This phenomenon, reminiscent of the play of colours seen in oil slicks, is a distinctive feature of bismuth crystals, adding an aesthetic allure to these naturally occurring formations. The oxidised layer refracts light, producing a spectrum of hues that enhances the visual appeal of bismuth specimens, making them highly sought-after in both scientific and collector communities. In the creation of these samples, bismuth undergoes a carefully orchestrated process in a lab, where it is melted and allowed to cool, growing into its own unique natural crystal shapes. At first glance, these specimens may resemble something out of a 3D printer, but in reality, this is the natural way the mineral grows as it cools and assumes its unique crystal lattice. No molding or trickery is involved—this showcases the inherent beauty of natural elements, similar to how amethyst and citrine grow into long, thin pointed crystals, pyrite forms perfect cubes, and optic calcite takes the shape of offset parallelograms. Bismuth, a chemical element denoted by the symbol Bi with the atomic number 83, is characterized by its distinctive metallic hue, setting it apart from other elements. What makes bismuth particularly intriguing is its relatively low melting point, making it one of the rare elemental metals that can melt at moderate temperatures. The crystal structure of bismuth is exceptionally unique, forming stunning stepped hopper-shaped crystals during solidification. This phenomenon results from a higher growth rate at the crystal’s edges compared to its interior. While bismuth doesn’t play a recognized biological role in living organisms, it has various applications in the human world. Bismuth subsalicylate, for example, is employed in medications targeting digestive system disorders, while bismuth oxychloride serves as a component in cosmetics. Bismuth’s utility extends to alloys as well, often combined with other metals to create low-melting alloys used in fire sprinkler systems and soldering applications. Despite its usefulness, bismuth is relatively uncommon in nature, typically extracted as a byproduct during the processing of lead and copper ores. In terms of toxicity, bismuth is generally less toxic than some other heavy metals, and in its oxidized form, it poses no risk. Cases of bismuth poisoning are rare. Bismuth’s intriguing characteristics, both in terms of its appearance and applications, make it a noteworthy element in the periodic table.
- Ultimate Guide to the Par Puca French Designer Beads Range - May 28, 2024
- Meet Travis, the New Wizard (sort of) at Beads N Crystals! - January 3, 2024
- Unveiling the Brilliance: Stainless Steel Findings and the Hypoallergenic Advantage - December 11, 2023